
Two years after the launch of our PowerSwap NucleusTM battery pack, we’ve seen a lot of success stories from our customers who’ve upgraded from their original Sealed Lead Acid (SLA) power packs. Although Lithium is something we are all using day-to-day in our personal lives in our cell phones, laptop and almost any other rechargeable device we have, we still get a lot of questions from our customers and readers about some of the more basic aspects of our power packs.
- How exactly does it work?
- What advantages does Lithium have over SLA?
- Why does Newcastle use a different Lithium chemistry (LiFePo4)?
For the record, Lithium as a battery chemistry is credited to John Goodenough, who first conceived of using Lithium at the University of Texas in 1996, where he still teaches mechanical engineering and materials science. More than any other innovation, the advent of Lithium as a battery chemistry arguably allowed for the advances in shrinking the size and durability of many of our devices in what would become the Digital Age.
How does a Lithium-ion battery Work?
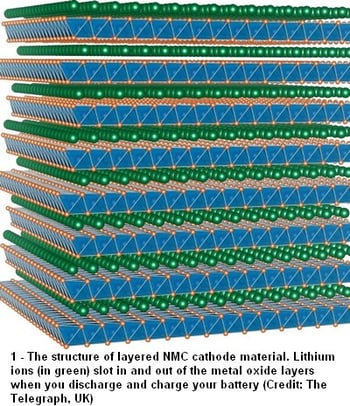 The standard Lithium-Ion (LI-ion) battery that you use every day in your cell phone, laptop, mobile scanner, power tools and perhaps even your forklift or your car is comprised of five components:
The standard Lithium-Ion (LI-ion) battery that you use every day in your cell phone, laptop, mobile scanner, power tools and perhaps even your forklift or your car is comprised of five components:
- Cathode
- Anode
- Separator
- Current Collectors
- Electrolyte
The lithium in the battery is stored in the anode and cathode, while the electrolyte is the medium used by the positively-charged lithium to travel. When you are charging the battery, the cathode (which is a positive electrode) releases its lithium ions which pass through the electrolyte and the separator before they arrive at the anode (which is a negative electrode). When you turn on the device being powered from your battery, the flow is reversed, with the lithium ions now moving back to the cathode from the anode. This is the movement that generates the electrons that power your device.
The travel path for the ions is reversed when you are discharging the battery (i.e. when you are powering a device with it) as the lithium particles lose their positive charge.
Simple, right?
Well, we won’t get much more technical than that. But in reality, the entire process is all managed within those five elements listed above, and it gives Lithium a big advantage over the SLA batteries that have been in use for over a hundred years.
Why Lithium over SLA?
It’s a great question. We asked that ourselves three years ago when we started down the path of creating our own power pack. Our existing SLA products are basically off the shelf products that we assemble ourselves and mount inside our own manufactured box to keep them all together and organized in one place. But the SLA solution always had a number of flaws, some of which we heard often enough from our customers to help us speed up the process for finding an alternative:
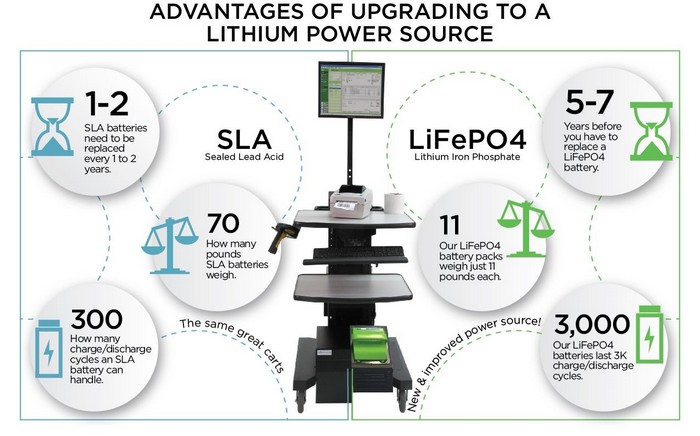
- They’re HEAVY - our most powerful SLA power pack adds 180 lbs to our cart. A typical small marine battery only 7 inches long and 6 inches tall can weight up to 35 lbs on its own. Our Lithium units, which are slightly bigger, weigh only 11lbs.
- They only have about 300 cycles – While some customers have been able to use their SLA batteries for up to 18 months and more, 300 cycles generally means 300 days of use with a recharge after each day. That is just about a year for a six-day/week facility. Lithium? About 3,000 cycles. Or about TEN YEARS if you are using and recharging six days/week.
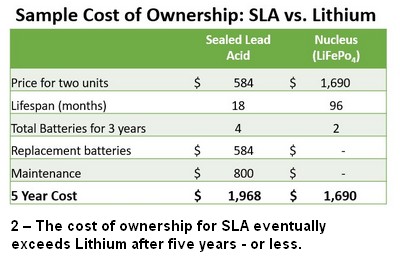
- Cost of Ownership – While SLA chemistry is the cheapest option out there at the time of purchase, we learned that the “Total Cost of Ownership” for SLA can outpace that of our Lithium units in five years or less. The cost of replacing the SLA unit inside the power pack, the time spent doing it and any downtime resulting from it all surpass the cost, and utility, of the Lithium after 3-5 years depending on your work environment.
The chemistry of the Lithium option has obvious advantages over SLA on its own merits. But we also realized that once we took out all the weight of the SLA and were dealing with a unit that is only eleven pounds, it meant we could play with the form factor too. So we also made it swappable! Basically, we created an option to have a power “platform” with a single battery that could be used interchangeably across all of your powered carts of any type. And units that are on the floor 24/7 can keep moving without shutting down the workstation by loading a freshly recharged battery before removing the spent one.
Why LiFePo4 instead of Li-Ion?
The chemistry “name” you see for various Lithium batteries is basically a reference to the material being used in the cathode element of the battery. The typical Li-Ion battery is usually a Lithium Cobalt Oxide (LiCoO2). When we sought out people who really know this stuff better than we do, we discovered the option of LiFePo4 and learned of a number of advantages:

- Lower cost – this was an immediate advantage in our eyes, because Lithium was already far more expensive up front than SLA, so using LiFePo4 had a major advantage when we did our “cost of ownership” modeling.
- Safer – Unlike those Lithium batteries you always hear about catching fire at the most inconvenient times, LiFePo4 is much safer. Not only does it not decompose at high temperatures, but it also has much higher level of stability than Cobalt-based cathodes, and is much harder to ignite in the case of mishandling or abuse.
- Long-term stability - LiFePo4 has a much slower rate of capacity loss than other Cobalt- based batteries, which means that it will retain a higher percentage of its watt-hours than other chemistries. It also has a much slower degradation rate when stored in a fully charged state, making it ideal as a “standby” power option
- Less Toxic – The phosphates used in LiFePo4 contribute to a safer product. The concern’s over cobalt entering then environment through improper disposal is not present, and it has a lower chance of experiencing any thermal runaway, which is a chemical process that can result in overheating and sometimes exploding batteries.
But it’s Not for Everyone - An SLA battery still retains a place
As you can see, Lithium batteries, and specifically our chosen chemistry, LiFePo4 has a number of major advantages over other traditional SLA batteries, nickel-based rechargeables and even the typical Lithium-Ion units we use every day at home and work.
But SLA still retains a place in our business, because depending on the solution your business needs, it may sometimes just be the best option. This is common for our clients that generally have lower volume use, or just have a single shift on the floor or don’t have a fleet of carts where the swappability of the battery would provide an obvious benefit.
Have any questions about battery technology? Any experiences you care to share? We’re always interested to hear your ideas, let us know!










